Abstract
Background: The ongoing Phase 1/2 HGB-206 study (NCT02140554) of LentiGlobin for SCD (bb1111) GT uses a modified human β-globin gene that expresses an anti-sickling hemoglobin (HbA T87Q). The relationships between biological outcomes, clinical outcomes, and clonality in the initial cohort (Group A) and the cohort treated after substantial changes were made to the study protocol and manufacturing process to improve clinical benefit (Group C) are presented here.
Methods: Patients (pts; ≥18 in Group A and ≥12-≤50 yrs in Group C) with SCD and recurrent severe vaso-occlusive events (VOEs), overt stroke, or tricuspid regurgitant jet velocity of >2.5 m/s, were enrolled. The initial protocol (cell collection and target busulfan dose) and manufacturing process in Group A was modified to improve cell dose, transduction efficiency, HbA T87Q expression, and clinical benefit. CD34+ cells (collected by bone marrow [BM] harvesting in Group A and plerixafor mobilization/apheresis in Group C) were transduced with BB305 lentiviral vector (LVV). LentiGlobin was infused after myeloablative busulfan conditioning. Transduction, SCD-related outcomes, clonality, and safety were assessed; data are median (min-max) unless otherwise stated.
Results: As of 17 February 2021, there were 61.5 (55.5-66.1) months of follow-up post-LentiGlobin infusion in Group A (n=7) and 17.3 (3.7-37.6) months in Group C (n=35). After protocol and manufacturing modifications, median drug product vector copy number (DP VCN) and transduction efficiency were increased in Group C (3.7 c/dg with 80.3% transduced cells) compared with Group A (0.6 c/dg with 27.7% transduced cells). Peripheral blood (PB) VCN stabilized by Month 6 post-infusion and was sustained throughout follow up in both groups; however, the median PB VCN was correspondingly higher in Group C than in Group A (1.45 c/dg vs 0.09 c/dg). A higher DP VCN, %LVV+, and PB VCN in Group C generated increased HbA T87Q of 5.2 (2.6-8.8) g/dL (n=30) compared with HbA T87Q of 0.5 (0.1-1.8) g/dL (n=7) in Group A at Month 6. This was associated with near pancellular expression of HbA T87Q at ≥6 months post-infusion in Group C with a mean of 87% of red blood cells containing β A-T87Q by 18 months (n=14).
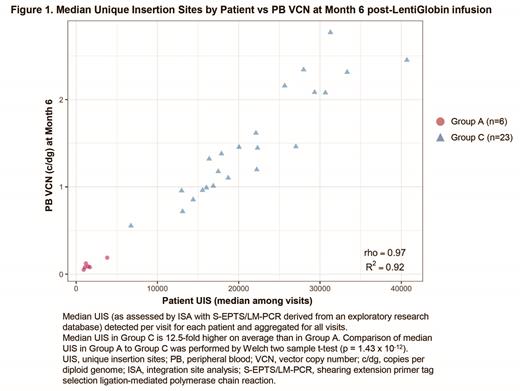
Group C featured significantly higher median unique insertion sites (UIS) than Group A (p = 1.43 x 10 -12;Fig 1), consistent with increased polyclonality. Critically, median UIS also correlated strongly with PB VCN (Spearman rho = 0.97; Fig 1) and HbA T87Q at Month 6 post-infusion and was associated with improved clinical efficacy in Group C, with complete resolution of severe VOEs and near normal levels of key hemolysis markers.
In Group C, the only treatment (tx) emergent serious adverse events (TESAEs) reported in >1 pt were abdominal pain, nausea, opioid withdrawal syndrome, and vomiting (n=2, 5.7% each). No events of malignancy were reported in Group C. One event of sudden death, considered unlikely related to LentiGlobin, occurred >18 months post-tx in a patient with significant baseline SCD-related cardiopulmonary disease. In Group A, the most common TESAE was sickle cell anemia with crisis (n=4, 57%). Two events of acute myeloid leukemia (AML) were reported in Group A pts at 3 and 5 years post-tx, both of which were considered unlikely related to the LVV. Both pts had classic AML driver mutations identified post-diagnosis. One pt died of AML and the second pt is receiving therapy for AML.
The modifications made in Group C are anticipated to reduce risk of AML. To monitor safety, BM and PB will be screened for the presence of AML driver mutations prior to treatment, and patients already treated will have regular cytogenetic screening in addition to BB305 LVV integration site analysis.
Summary: Alterations to the protocol and manufacturing process in HGB-206 resulted in improved cell dose, transduction efficiency, HbA T87Q expression, and clinical outcomes in Group C compared with Group A. Polyclonality was strongly correlated to PB VCN and HbA T87Q production, indicating that superior engraftment of LVV-transduced cells leads to favorable clinical outcomes. The safety profile post-LentiGlobin for all treated patients with SCD remains generally consistent with the risks of autologous stem cell transplant, myeloablative busulfan conditioning, and underlying SCD.
Thompson: Baxalta: Research Funding; Biomarin: Research Funding; bluebird bio, Inc.: Consultancy, Research Funding; Celgene/BMS: Consultancy, Research Funding; CRISPR Therapeutics: Research Funding; Vertex: Research Funding; Editas: Research Funding; Graphite Bio: Research Funding; Novartis: Research Funding; Agios: Consultancy; Beam: Consultancy; Global Blood Therapeutics: Current equity holder in publicly-traded company. Kwiatkowski: Bluebird Bio: Other: Consultancy Fees; Imara: Other: Consultancy Fees; Celgene: Honoraria; Silence Therapeutics: Honoraria; Agios: Honoraria; ApoPharma: Research Funding; Novartis: Research Funding; Bluebird Bio: Research Funding; Sangamo: Research Funding; Terumo BCT: Research Funding. Aygun: National Heart, Lung, Blood Institute: Research Funding; Global Blood Therapeutics: Consultancy; National Institute of Nursing Research: Research Funding; Patient Centered Outcomes Research Institute: Research Funding; bluebird bio, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding. Schmidt: GeneWerk GmbH, Heidelberg, Germany: Current equity holder in publicly-traded company; German Cancer Research Center, Heidelberg, Germany: Current Employment. Pierciey: bluebird bio, Inc.: Current Employment, Current equity holder in publicly-traded company. Whitney: bluebird bio, Inc.: Current Employment, Current equity holder in publicly-traded company. Rogers: bluebird bio, Inc.: Current Employment, Current equity holder in publicly-traded company. Nnamani: bluebird bio, Inc.: Current Employment, Current equity holder in publicly-traded company. Foos: bluebird bio, Inc.: Current Employment, Current equity holder in publicly-traded company. Miller: bluebird bio, Inc.: Current Employment, Current equity holder in publicly-traded company. Zhang: bluebird bio, Inc.: Current Employment, Current equity holder in publicly-traded company. Lynch: bluebird bio, Inc.: Current Employment, Current equity holder in publicly-traded company. Walters: Vertex pharmaceuticals: Consultancy; Ensoma, Inc.: Consultancy; BioLabs, Inc: Consultancy; AllCells, Inc: Consultancy. Kanter: Fulcrum Therapeutics, Inc.: Consultancy; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Forma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Agios: Honoraria, Membership on an entity's Board of Directors or advisory committees; Beam: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Graphite Bio: Consultancy; GuidePoint Global: Honoraria; Fulcrum Tx: Consultancy. Bonner: bluebird bio, Inc.: Current Employment, Current equity holder in publicly-traded company.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal